What is organic Chemistry?..
Basic Concepts of Organic Chemistry
– Definition and scope of organic chemistry
– Tetravalency of carbon
– Shapes of organic molecules
– Structural representation of organic compounds (Bond-line, Newman, Sawhorse, etc.)
**Hydrocarbons**
– Classification
– Alkanes
– Alkenes
– Alkynes
– Aromatic hydrocarbons (Benzene and its derivatives)
**Functional Groups**
– Alcohols, Phenols, and Ethers
– Aldehydes and Ketones
– Carboxylic Acids and their derivatives (Acid chlorides, esters, amides, etc.)
– Nitro compounds
– Amines and Diazonium salts
**Nomenclature**
– IUPAC rules for naming organic compounds
– Common names and their conversion to IUPAC names
**Isomerism**
– Structural isomerism
– Stereoisomerism (Geometric and Optical)
**Reactions**
– Substitution reactions
– Addition reactions
– Elimination reactions
– Condensation reactions
– Oxidation and Reduction reactions
**Mechanism of Organic Reactions**
– Types of reaction mechanisms (Electrophilic addition, Nucleophilic substitution, etc.)
– Elementary steps involved in reaction mechanisms
– Energy profile diagrams
**Important Named Reactions**
– Alkane reactions (e.g., Halogenation)
– Alkene reactions (e.g., Addition of Hydrogen halides, Hydration)
– Alkyne reactions (e.g., Hydrogenation)
– Reactions of Benzene (e.g., Nitration, Halogenation)
**Polymers**
– Classification of polymers
– Preparation and properties of polymers (Addition and condensation polymers)
**Biomolecules**
– Carbohydrates
– Proteins
– Lipids
– Nucleic Acids
Chemistry in Everyday Life**
– Drugs and their classification
– Chemicals in food
– Cleansing agents
**Environmental Chemistry**
– Greenhouse effect and global warming
– Acid rain
– Ozone depletion
This structured outline provides a clear overview of the organic chemistry syllabus for class 12th, facilitating systematic learning and understanding of the subject.
**Basic Concepts of Organic Chemistry**
– **Definition and Scope**: Organic chemistry is the study of carbon compounds, which are essential to life and have vast applications in various fields such as pharmaceuticals, materials science, and agriculture.

– **Tetravalency of Carbon**: Carbon has four valence electrons, allowing it to form stable covalent bonds with other atoms, including itself. This property enables the formation of diverse organic compounds.
– **Shapes of Organic Molecules**: Organic molecules can adopt different shapes depending on the arrangement of atoms and the types of bonds present. Understanding molecular geometry is crucial for predicting chemical behavior.
– **Structural Representation**: Various methods like bond-line notation, Newman projection, and Sawhorse projection are used to represent the three-dimensional structure of organic compounds in a simplified manner.

– **Hydrocarbons**
– **Classification**: Hydrocarbons are organic compounds composed exclusively of hydrogen and carbon atoms. They are classified into different types based on the types of carbon-carbon bonds present:
– **Alkanes**: Contain only single bonds between carbon atoms.
– **Alkenes**: Contain at least one carbon-carbon double bond.
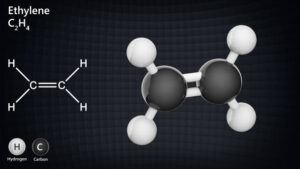
– **Alkynes**: Contain at least one carbon-carbon triple bond.
– **Aromatic Hydrocarbons**: Contain one or more benzene rings, characterized by alternating single and double bonds.
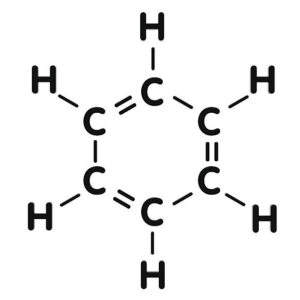
– **Functional Groups**
– **Alcohols, Phenols, and Ethers**: Organic compounds containing hydroxyl (-OH) groups are classified as alcohols (if the -OH group is attached to a saturated carbon), phenols (if the -OH group is attached to a benzene ring), and ethers (if the -OH group is bonded to two carbon atoms).
– **Aldehydes and Ketones**: Both aldehydes and ketones contain the carbonyl group (C=O), but aldehydes have the carbonyl group at the end of the carbon chain, while ketones have it within the chain.
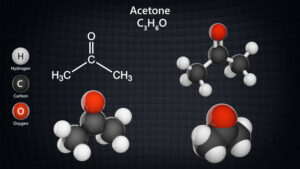
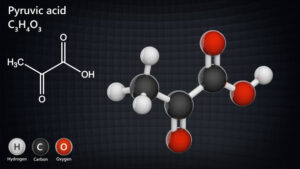
– **Carboxylic Acids and Derivatives**: Carboxylic acids have the carboxyl group (-COOH), while their derivatives include acid chlorides, esters, amides, and anhydrides.

– **Nitro Compounds**: Compounds containing the nitro functional group (-NO₂).
– **Amines and Diazonium Salts**: Amines contain the amino functional group (-NH₂), while diazonium salts are compounds containing the diazonium group (-N₂⁺X⁻).
– **Nomenclature**
– **IUPAC Rules**: The International Union of Pure and Applied Chemistry (IUPAC) provides systematic rules for naming organic compounds based on their structure, substituents, and functional groups.
– **Examples**: Naming organic compounds involves identifying the longest carbon chain, determining the parent functional group, and assigning locants to substituents according to priority rules.
– **Isomerism**
– **Structural Isomerism**: Isomers with the same molecular formula but different structural arrangements. This includes chain isomerism, positional isomerism, and functional group isomerism.
– **Stereoisomerism**: Isomers with the same connectivity of atoms but different spatial arrangements. This includes geometric isomerism (cis-trans isomerism) and optical isomerism (enantiomerism).
– **Reactions**
– **Substitution, Addition, Elimination, Condensation, Oxidation, and Reduction Reactions**: These are fundamental types of organic reactions involving the breaking and forming of bonds.
– **Examples**:
– Substitution: Replacement of one atom or group in a molecule with another atom or group. Example: Halogenation of alkanes.
– Addition: Addition of atoms or groups to a molecule without removing any part of it. Example: Hydrogenation of alkenes.
– Elimination: Removal of atoms or groups from a molecule to form a double or triple bond. Example: Dehydration of alcohols.
– Condensation: Two molecules combine to form a larger molecule with the elimination of a smaller molecule. Example: Esterification reaction.
– Oxidation: Increase in the oxidation state of carbon in a molecule, often involving the addition of oxygen or removal of hydrogen. Example: Oxidation of alcohols to aldehydes or carboxylic acids.
– Reduction: Decrease in the oxidation state of carbon in a molecule, often involving the addition of hydrogen or removal of oxygen. Example: Reduction of aldehydes to alcohols.
– **Mechanism of Organic Reactions**
– **Types of Reaction Mechanisms**: Different types of organic reactions proceed through specific mechanisms, such as electrophilic addition, nucleophilic substitution, and free radical substitution.
– **Examples**:
– Electrophilic Addition: Electrophiles attack the double bond of an alkene to form a single bond with one of the carbon atoms. Example: Addition of hydrogen halides to alkenes.
– Nucleophilic Substitution: Nucleophiles attack an electrophilic center, leading to the displacement of a leaving group. Example: SN1 and SN2 reactions.
– **Important Named Reactions**
– These are well-known organic reactions named after their discoverers or significant contributors. Examples include the Friedel-Crafts alkylation reaction, Grignard reaction, and Diels-Alder reaction.
– **Polymers**
– **Classification**: Polymers are large molecules composed of repeating structural units called monomers. They are classified into addition polymers (formed by the repeated addition of monomers) and condensation polymers (formed by the elimination of small molecules like water).
– **Preparation**: Polymers are synthesized through various polymerization techniques, including addition polymerization and condensation polymerization.
– **Examples**:
– Addition Polymerization: Formation of polyethylene from ethylene monomers.
– Condensation Polymerization: Formation of nylon-6,6 from adipic acid and hexamethylenediamine monomers.
– **Biomolecules**
– Organic molecules essential for life processes, including carbohydrates, proteins, lipids, and nucleic acids. They play vital roles in cell structure, energy storage, and genetic information transfer.
– **Examples**:
– Carbohydrates: Glucose, Fructose, Sucrose
– Proteins: Insulin, Hemoglobin, Enzymes
– Lipids: Triglycerides, Phospholipids, Steroids
– Nucleic Acids: DNA, RNA
– **Chemistry in Everyday Life**
– The application of organic chemistry principles to everyday products and processes, including pharmaceuticals, food additives, and household cleaners.
– **Examples**:
– Drugs and Their Classification: Analgesics (e.g., Aspirin), Antibiotics (e.g., Penicillin), and Antipyretics (e.g., Paracetamol)
– Chemicals in Food: Vitamins, Flavor Enhancers, and Preservatives
– Cleansing Agents: Soaps, Detergents, and Shampoos
Environmental Chemistry
Greenhouse Effect and Global Warming: The phenomenon where greenhouse gases, such as carbon dioxide and methane, trap heat in the Earth’s atmosphere, leading to a rise in global temperatures.
Acid Rain: Rainfall containing acidic components, primarily sulfuric and nitric acids, resulting from the atmospheric deposition of pollutants emitted by human activities.
Ozone Depletion: The thinning of the ozone layer in the Earth’s stratosphere due to the release of ozone-depleting substances, such as chlorofluorocarbons (CFCs) and halons, resulting in increased UV radiation reaching the Earth’s surface.