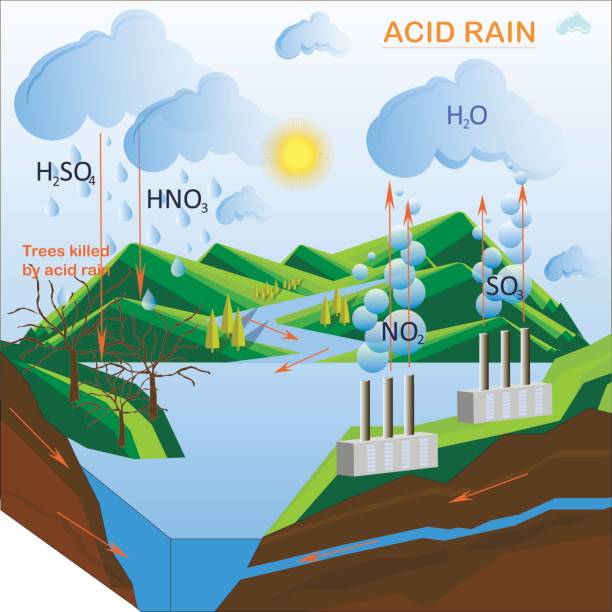
What is Acid Rain ?
Acid rain is a complex environmental issue that occurs when sulfur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere and undergo chemical reactions with water, oxygen, and other compounds to form sulfuric acid (H2SO4) and nitric acid (HNO3). These acids then fall to the Earth’s surface as rain, snow, fog, or dry particles.
Introduction
– Definition and background of acid rain
– Importance of understanding its causes and effects
Causes
– Natural sources
– Volcanic activity
– Biological processes
– Human activities
– Fossil fuel combustion
– Industrial emissions
– Transportation emissions
Formation Process
– Release of sulfur dioxide (SO2) and nitrogen oxides (NOx)
– Chemical reactions in the atmosphere
– Formation of sulfuric acid (H2SO4) and nitric acid (HNO3)
Effects
– Environmental impact
– Damage to forests and aquatic ecosystems
– Corrosion of buildings and infrastructure
– Health effects
– Respiratory issues
– Skin irritation
Mitigation
– Reduction of emissions
– Adoption of cleaner technologies
– International cooperation and regulations
Conclusion
– Recap of main points
– Call to action for addressing acid rain issue
Introduction:
Acid rain, a phenomenon that gained widespread recognition in the late 20th century, refers to the deposition of acidic substances from the atmosphere onto the Earth’s surface. Primarily caused by emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx), acid rain poses significant threats to ecosystems, infrastructure, and human health. Understanding its causes, mechanisms, and impacts is crucial for devising effective strategies to mitigate its effects.
Causes:
1. **Natural Sources:**
Natural processes such as volcanic eruptions and biological activities contribute to the release of sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere. Volcanic eruptions emit large quantities of sulfur dioxide (SO2), which can lead to the formation of sulfuric acid (H2SO4) when combined with atmospheric moisture. Similarly, biological processes such as decomposition and bacterial activities release sulfur-containing compounds, contributing to acid rain formation.
2. **Human Activities:**
The combustion of fossil fuels, particularly coal, oil, and natural gas, is the primary source of sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions. Industrial processes, including manufacturing, mining, and refining, also release substantial amounts of these pollutants into the atmosphere. Additionally, transportation activities, such as vehicle emissions from cars, trucks, and aircraft, contribute significantly to the release of nitrogen oxides (NOx) and sulfur dioxide (SO2).
Formation Process:
1. **Release of Pollutants:**
Sulfur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere during combustion processes, industrial activities, and transportation. These pollutants are emitted in large quantities, especially in regions with high levels of industrialization and vehicular traffic.
2. **Chemical Reactions:**
Once released into the atmosphere, sulfur dioxide (SO2) and nitrogen oxides (NOx) undergo complex chemical reactions with atmospheric constituents such as water vapor, oxygen, and other pollutants. These reactions result in the formation of sulfuric acid (H2SO4) and nitric acid (HNO3), which are highly soluble in water.
3. **Acid Deposition:**
The sulfuric acid (H2SO4) and nitric acid (HNO3) formed in the atmosphere are carried by winds and atmospheric currents over long distances. Eventually, these acids are deposited onto the Earth’s surface as acid rain, snow, fog, or dry particles. Acid deposition can occur both locally, near pollution sources, and regionally, affecting areas far from the emission sources.
Effects:
1. **Environmental Impact:**
Acid rain has detrimental effects on various ecosystems, including forests, lakes, rivers, and soils. It leaches essential nutrients such as calcium, magnesium, and potassium from the soil, leading to nutrient depletion and soil acidification. In forests, acid rain damages foliage, weakens tree roots, and inhibits seed germination, ultimately impacting forest health and biodiversity.
2. **Harm to Aquatic Ecosystems:**
Acid rain can acidify bodies of water such as lakes, rivers, and streams, affecting aquatic organisms such as fish, amphibians, and aquatic plants. It disrupts the balance of pH levels in aquatic ecosystems, making it difficult for organisms to survive and reproduce. Acidification also mobilizes toxic metals such as aluminum, which can accumulate in aquatic organisms and further exacerbate ecosystem degradation.
3. **Corrosion of Buildings and Infrastructure:**
The corrosive nature of acid rain accelerates the deterioration of buildings, monuments, bridges, and other infrastructure made of stone, metal, or concrete. It causes erosion of building materials, corrosion of metal structures, and discoloration of surfaces, leading to structural damage and aesthetic degradation. Acid rain-induced corrosion poses significant challenges for maintenance and preservation efforts, especially for historical and cultural landmarks.
4. **Health Effects:**
Exposure to sulfur dioxide (SO2) and nitrogen oxides (NOx) emitted from combustion sources can have adverse effects on human health. Inhalation of these pollutants can irritate the respiratory system, exacerbate asthma and other respiratory conditions, and increase susceptibility to respiratory infections. Additionally, direct contact with acid rain or its components can cause skin irritation, dermatitis, and other skin disorders.
Mitigation:
1. **Reduction of Emissions:**
Implementing stringent emission standards and adopting cleaner technologies are essential strategies for reducing sulfur dioxide (SO2) and nitrogen oxides (NOx) emissions from industrial processes, power plants, and vehicles. Technologies such as flue gas desulfurization (FGD) and selective catalytic reduction (SCR) can effectively reduce pollutant emissions from coal-fired power plants and industrial facilities.
2. **Alternative Energy Sources:**
Transitioning to renewable energy sources such as wind, solar, and hydropower can reduce reliance on fossil fuels and lower emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx). Renewable energy technologies offer sustainable alternatives to conventional energy sources, contributing to cleaner air and mitigating the impacts of acid rain on the environment and human health.
3. **International Cooperation and Regulations:**
International agreements and regulations play a crucial role in addressing transboundary air pollution and mitigating the impacts of acid rain on a global scale. Agreements such as the Kyoto Protocol and the Paris Agreement aim to reduce greenhouse gas emissions, including sulfur dioxide (SO2) and nitrogen oxides (NOx), through coordinated efforts among nations. Additionally, regional initiatives and collaborative projects facilitate information sharing, technology transfer, and capacity building to address acid rain and other environmental challenges.
4. **Restoration Efforts:**
Implementing measures to restore damaged ecosystems, such as reforestation, soil remediation, and habitat restoration, can help mitigate the long-term effects of acid rain. Restoring natural habitats and ecosystem functions enhances resilience to environmental stressors and promotes biodiversity conservation. Furthermore, incorporating sustainable land management practices and ecosystem-based approaches can improve ecosystem health and ecosystem services, contributing to the overall well-being of communities and ecosystems affected by acid rain.
Conclusion:
Acid rain remains a significant environmental challenge that requires concerted efforts at the local, national, and international levels to address its causes and mitigate its impacts. By reducing emissions, adopting cleaner technologies, promoting renewable energy sources, and restoring damaged ecosystems, we can minimize the adverse effects of acid rain on the environment, infrastructure, and human health. Continued research, monitoring, and collaboration are essential for developing effective strategies and policies to combat acid rain and ensure a sustainable future for generations to come.