What is Nitrogen Cycle?
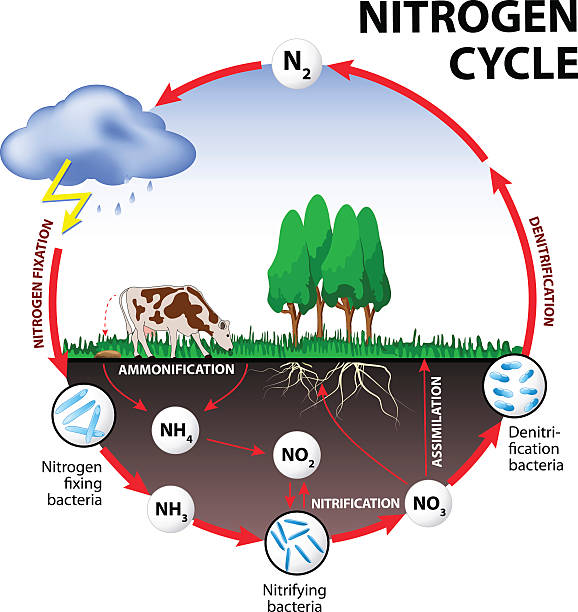
The nitrogen cycle is a fundamental process that governs the movement and transformation of nitrogen in various forms throughout the biosphere. Nitrogen, a vital element for all living organisms, plays a crucial role in the structure of proteins, nucleic acids, and other essential biomolecules. This cycle encompasses a series of interconnected biological, chemical, and physical processes that continuously recycle nitrogen between the atmosphere, soil, water bodies, and living organisms.
– Introduction to Nitrogen
– Importance of nitrogen
– Abundance in the atmosphere
– Forms of nitrogen
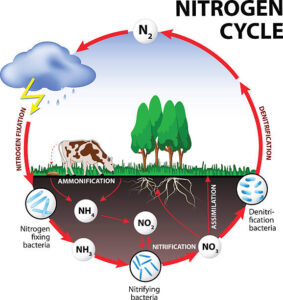
– Nitrogen Fixation
– Definition
– Natural processes (lightning, nitrogen-fixing bacteria)
– Industrial processes (Haber-Bosch process)
– Ammonification
– Conversion of organic nitrogen to ammonium by decomposers
– Role of bacteria and fungi
– Release of ammonium into the soil
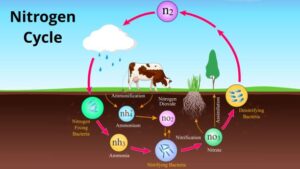
– Nitrification
– Conversion of ammonium to nitrite and then to nitrate
– Nitrifying bacteria (Nitrosomonas and Nitrobacter)
– Conditions necessary for nitrification
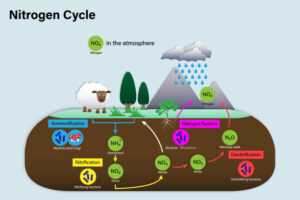
– Assimilation
– Uptake of nitrogen by plants and other organisms
– Conversion of nitrate and ammonium into organic nitrogen compounds (amino acids, proteins)
– Role of plants and microbes in assimilation
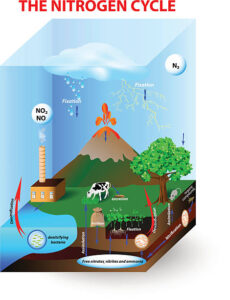
– Denitrification
– Conversion of nitrate into nitrogen gas
– Denitrifying bacteria (Pseudomonas, Paracoccus)
– Anaerobic conditions required for denitrification
– Nitrogen Losses
– Leaching
– Volatilization
– Runoff
– Human Impacts on the Nitrogen Cycle
– Agriculture (fertilizer use, nitrogen runoff)
– Industrial processes (combustion, nitrogen emissions)
– Effects on ecosystems and water quality

– Nitrogen Cycle in Aquatic Environments
– Similarities and differences with terrestrial nitrogen cycle
– Importance of nitrogen in aquatic ecosystems
– Impact of human activities on aquatic nitrogen cycle
– Conclusion
– Summary of key points
– Importance of understanding nitrogen cycle for environmental management
– Future directions in nitrogen cycle research and management
The Nitrogen Cycle: Understanding Nature’s Essential Element
The nitrogen cycle is a fundamental process that governs the movement and transformation of nitrogen in various forms throughout the biosphere. Nitrogen, a vital element for all living organisms, plays a crucial role in the structure of proteins, nucleic acids, and other essential biomolecules. This cycle encompasses a series of interconnected biological, chemical, and physical processes that continuously recycle nitrogen between the atmosphere, soil, water bodies, and living organisms.
Introduction to Nitrogen:
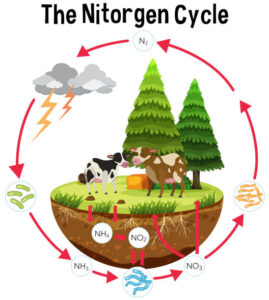
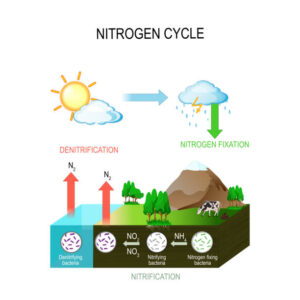
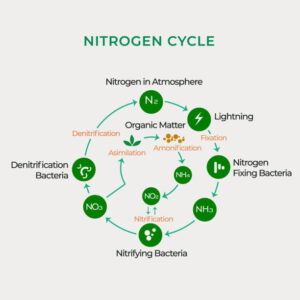
Nitrogen constitutes approximately 78% of the Earth’s atmosphere, primarily in the form of dinitrogen gas (N2). Despite its abundance, atmospheric nitrogen is largely inaccessible to most living organisms in this molecular form. Instead, nitrogen must undergo transformations into reactive nitrogen compounds, such as ammonia (NH3), nitrate (NO3-), and ammonium (NH4+), to become biologically useful.
Nitrogen Fixation:
Nitrogen fixation is the process by which atmospheric nitrogen is converted into ammonia or ammonium ions, which can be utilized by living organisms. This conversion is facilitated by nitrogen-fixing bacteria and archaea, as well as through abiotic processes like lightning. Additionally, industrial processes, such as the Haber-Bosch process, produce ammonia for the production of fertilizers, significantly impacting the global nitrogen cycle.
Ammonification:
Following the death and decomposition of organic matter, nitrogen is released in the form of ammonium ions through a process known as ammonification. Decomposers such as bacteria and fungi play a critical role in breaking down organic nitrogen-containing compounds into ammonium, which subsequently becomes available for uptake by plants and other organisms.
Nitrification:
Nitrification involves the oxidation of ammonium to nitrite and then to nitrate by nitrifying bacteria. The first step is carried out by ammonia-oxidizing bacteria, while the second step is performed by nitrite-oxidizing bacteria. These microbial processes primarily occur in aerobic environments, such as soils and aquatic systems, where oxygen is present. Nitrification plays a vital role in converting ammonia, a form of nitrogen toxic to many organisms, into nitrate, which is readily taken up by plants as a nutrient.
Assimilation:
Plants and other organisms assimilate nitrogen by incorporating inorganic nitrogen compounds, such as nitrate and ammonium, into organic molecules such as amino acids and proteins. Nitrogen assimilation is a fundamental process for the synthesis of biomolecules essential for growth, development, and reproduction. Plants acquire nitrogen from the soil through their roots, while animals obtain nitrogen by consuming plants or other organisms.
Denitrification:
Denitrification is the microbial process by which nitrate or nitrite is reduced to nitrogen gas or nitrous oxide under anaerobic conditions. Denitrifying bacteria carry out this process in environments where oxygen is limited or absent, such as waterlogged soils and sediments. Denitrification acts as a natural nitrogen removal mechanism, returning nitrogen to the atmosphere and completing the nitrogen cycle.
Nitrogen Losses:
Various processes result in the loss of nitrogen from ecosystems, including leaching, volatilization, and runoff. Leaching occurs when nitrate is washed out of the soil into groundwater, potentially contaminating drinking water sources. Volatilization refers to the conversion of nitrogen compounds into gaseous forms, such as ammonia, which can contribute to air pollution and acid deposition. Runoff carries nitrogen-containing compounds into water bodies, leading to eutrophication, algal blooms, and hypoxic conditions detrimental to aquatic ecosystems.
Human Impacts on the Nitrogen Cycle:
Human activities have significantly altered the global nitrogen cycle, primarily through agricultural practices, industrial processes, and combustion of fossil fuels. The widespread use of nitrogen-based fertilizers has increased nitrogen inputs to terrestrial and aquatic ecosystems, leading to environmental problems such as soil degradation, water pollution, and biodiversity loss. Industrial nitrogen fixation and combustion processes contribute to atmospheric nitrogen deposition, which can affect air quality, climate, and ecosystem dynamics.
Nitrogen Cycle in Aquatic Environments:
The nitrogen cycle in aquatic ecosystems follows similar processes as terrestrial environments but with some notable differences. Aquatic systems receive nitrogen inputs from various sources, including atmospheric deposition, surface runoff, and biological nitrogen fixation by aquatic organisms. Excessive nitrogen loading can lead to nutrient enrichment, algal blooms, and oxygen depletion, resulting in ecological imbalances and negative impacts on water quality and aquatic biodiversity.
Conclusion:
In conclusion, the nitrogen cycle is a complex and dynamic process that sustains life on Earth by recycling nitrogen in various forms essential for biological functioning. Understanding the intricacies of the nitrogen cycle is crucial for managing ecosystems, conserving natural resources, and mitigating the adverse effects of human activities on the environment. By adopting sustainable practices and promoting stewardship of nitrogen resources, we can strive towards a more resilient and balanced nitrogen cycle that supports the health and integrity of ecosystems worldwide.