Structure of Atom:
Outline of Structure of Atom:
1. Introduction to the Atom:
– Definition of an atom
– Importance of understanding atomic structure
2. Historical Development:
– Contributions of early philosophers (Democritus, Aristotle)
– Discovery of subatomic particles (electron, proton, neutron)
– Key experiments (Thomson’s cathode ray tube, Rutherford’s gold foil experiment)
3. Modern Atomic Theory:
– Bohr’s model of the atom
– Quantum mechanical model
– Electron configuration and energy levels
4. Subatomic Particles:
– Electron:
– Charge, mass, and location
– Role in chemical bonding
– Proton:
– Charge, mass, and location
– Role in determining element identity
– Neutron:
– Charge, mass, and location
– Role in stabilizing the nucleus
5. Atomic Structure:
– Nucleus:
– Composition (protons and neutrons)
– Size and density
– Electron Cloud:
– Probability distribution of electrons
– Orbitals and electron shells
6. Isotopes and Atomic Mass:
– Definition of isotopes
– Calculation of average atomic mass
– Importance of isotopes in various fields (medicine, archaeology, etc.)
7. Atomic Number and Mass Number:
– Definitions and symbols
– Relationship between atomic number, mass number, and number of subatomic particles
8. Nuclear Reactions:
– Types of nuclear reactions (fusion, fission)
– Energy release and applications (nuclear power, nuclear weapons)
9. Applications of Atomic Structure:
– Chemistry and chemical reactions
– Medicine (radiation therapy, diagnostic imaging)
– Materials science (semiconductors, nanotechnology)
10. Future Directions:
– Advances in understanding atomic structure
– Potential applications and implications (quantum computing, particle physics)
Structure of Atom:
1. Introduction to the Atom:
Definition of an atom:
An atom is the smallest particle of an element that may or may not exist independently and takes part in chemical reactions is called as an atom. It consists of a nucleus, containing protons and neutrons, surrounded by a cloud of electrons.

Importance of understanding atomic structure:
Understanding atomic structure is crucial in various fields such as chemistry, physics, and materials science. It forms the basis for explaining chemical reactions, understanding the behavior of matter, and developing technologies.
2.Historical Development:
Contributions of early philosophers:
Democritus proposed the idea of indivisible particles called “atoms,” while Aristotle believed in the existence of continuous matter. These early ideas laid the groundwork for modern atomic theory.
Discovery of subatomic particles:
Through experiments and observations, scientists discovered subatomic particles such as electrons, protons, and neutrons.

Key experiments:
Thomson’s cathode ray tube experiment demonstrated the existence of electrons, while Rutherford’s gold foil experiment revealed the structure of the atom’s nucleus.
3.Modern Atomic Theory:
Bohr’s model of the atom:
Niels Bohr proposed a model where electrons orbit the nucleus in specific energy levels or shells. This model explained the stability of atoms and the emission of spectral lines.
Quantum mechanical model:
The quantum mechanical model describes the behavior of electrons as both particles and waves. It involves probability distributions of finding electrons in specific regions around the nucleus.
Electron configuration and energy levels:
Electrons occupy specific energy levels and sublevels within an atom, dictated by quantum numbers. Understanding electron configuration is essential for predicting chemical properties and reactivity.
4.Subatomic Particles:
Electron:
Negatively charged, low mass particle orbiting the nucleus. Electrons play a crucial role in chemical bonding and determining the behavior of atoms in reactions.
Proton:
Positively charged particle located in the nucleus. The number of protons determines the element’s identity.
Neutron:
Neutral particle located in the nucleus. Neutrons stabilize the nucleus and contribute to the atom’s mass.
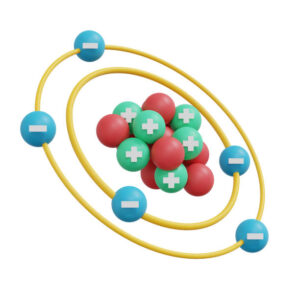
5.Atomic Structure:
Nucleus:
Central part of the atom containing protons and neutrons. It is small in size but contains most of the atom’s mass.
Electron Cloud:
Region surrounding the nucleus where electrons are likely to be found. The electron cloud is described by probability distributions called orbitals, which represent the different energy levels and sublevels.
6.Isotopes and Atomic Mass:
Definition of isotopes:
Isotopes are atoms of the same element with different numbers of neutrons. They have the same atomic number but different mass numbers.
Calculation of average atomic mass:
Average atomic mass is calculated based on the relative abundance of each isotope and their respective masses.
Importance of isotopes:
Isotopes have various applications in fields such as medicine (e.g., radioisotopes for imaging and treatment), archaeology (carbon dating), and environmental science.
7. Atomic Number and Mass Number:
Definitions and symbols:
Atomic number (Z) represents the number of protons in an atom, while mass number (A) represents the total number of protons and neutrons.
Relationship between atomic number, mass number, and number of subatomic particles:
The atomic number determines the element’s identity, while the mass number indicates the atom’s mass. The number of protons equals the atomic number, and the sum of protons and neutrons equals the mass number.
8. Nuclear Reactions:
Types of nuclear reactions:
Fusion involves the merging of atomic nuclei to form a heavier nucleus, releasing vast amounts of energy. Fission involves the splitting of a heavy nucleus into smaller nuclei, also releasing energy.
Energy release and applications:
Nuclear reactions release significant amounts of energy, which can be harnessed for various applications such as power generation, medical treatments, and weaponry.
9.Applications of Atomic Structure:
Chemistry and chemical reactions:
Atomic structure influences chemical properties and reactions, including bonding behavior and reactivity.
Medicine:
Understanding atomic structure is crucial for medical applications such as radiation therapy, diagnostic imaging (e.g., MRI, PET scans), and drug developmen.
Materials science:
Atomic structure affects the properties and behavior of materials, leading to advancements in fields like semiconductors, nanotechnology, and metallurgy.
10.Future Directions:
Advances in understanding atomic structure:
Ongoing research aims to further elucidate atomic structure and behavior at the quantum level, potentially leading to new discoveries and technologies
Potential applications and implications:
Advancements in atomic structure understanding could lead to breakthroughs in fields such as quantum computing, materials design, and energy generation, shaping the future of science and technology
Great explanation